Premise 1: The Law of Conservation of Mass-Energy cannot be suspended in order to all for evolution to be true
True science has always been founded on certain verifiable truths or axioms that are free from personal interpretation. These axioms have been empirically tested and repeated to the point of certainty and are called the Laws of Science. These Laws compose the Mount Rushmore of verified scientific truth.
The McGraw-Hill Dictionary of Scientific and Technical Terms defines a scientific law as:
“a regularity which applies to all members of a broad class of phenomena”
The chief characteristics of a Law of Science are:
- As a scientist takes care to make sure that the law applies to the scenario in question, the law will always hold true
- A Law of Science has graduated from theory, or hypothesis because it has been observed, tested and repeated throughout the history of known science and has been true without exception.
The Laws of Science are rooted in physical and biological reality—what makes them Laws is that no contradictory observations or evidence has ever been found that dispute them.
One of the first laws of physics children learn while in middle school is the Law Of Conservation of Mass discovered by Antoine Lavoisier in 1789 and they stated it like this “Matter can neither be created or destroyed”.
Wikipedia defines the Law of Conservation of Mass this way:
In physics and chemistry, the Law of Conservation of Mass states that for any system closed to all transfers of matter and energy, the mass of the system must remain constant over time, as the system’s mass cannot change, so quantity can neither be added nor be removed.
Wikipedia Conservation of Mass
An example of this would be a candle that burns in a closed room. The matter or wax would melt (changing forms) but the mass of the matter (wax) would remain the same.
The 1st Law of Thermodynamics is similar to the Law of Mass Conservation but in regards to energy, it reads:
“Energy is neither created nor destroyed, it just changes forms”
If you ask Google “How the Solar System formed and evolved” you will find answers like these:
The formation and evolution of the Solar System began 4.5 billion years ago with the gravitational collapse of a small part of a giant molecular cloud. Most of the collapsing mass collected in the center, forming the Sun, while the rest flattened into a protopanetary disk out of which the planets, moons, asteroids, and other small Solar System bodies formed.
Wikipedia
An extremely dense point exploded with unimaginable force, creating matter and propelling it outward to make the billions of galaxies of our vast universe. Astrophysicists dub this titanic explosion the Big Bang.
Exploratorium.edu
Most of the hydrogen and helium in the Universe were created in the moments after the Big Bang. Heavier elements came later. The explosive power of supernovae creates and dispersed a wide range of elements.
NASA.gov
However, upon close inspection of these popularized explanations you soon see them collide head-on with the fixed Law of Mass Conservation.
The Theory of Evolution and the Big Bang theory try to explain the origin of the universe using natural means and mechanisms without a Creator. The Big Bang theory claims that all the matter in the Universe initially was condensed in a sphere smaller than dot or period on a page. That sphere exploded and helps to explain why the Universe, according to many cosmologists, appears to be expanding. However, the theory offers no explanation for the origin of the matter supposedly condensed in the small sphere.
Inflation takes a very small universe and produces from it a very big universe. But inflation by itself does not explain where that very small universe came from
Evolutionist Alan Guth; Cosmologist and Physics Professor M.I.T.
Challenge Question : If matter cannot be created or destroyed in a closed system, where and how did the “molecules” that made up the molecular cloud or the “collapsing mass” as mentioned in these explanations originate or come from?
- Was it spontaneously generated from nothing ?
- Was the original matter eternal?
- Was the original matter created by God?
Premise 2: The Theory of Evolution violates the 2nd Law of Thermodynamics
The 2nd Law of Thermodynamics states fundamentally that all molecular arrangements tend to become less organized over time. That no energy conversion is 100% efficient, everything runs out of useable energy.
For example, when a car converts chemical energy to mechanical energy, some of the energy is lost as heat during the conversion. This heat eventually dissipates into space. While this dissipated heat is till a form of energy, it can no longer be readily recaptured and used as a functional source of energy.
Physical systems (such as the human body) need energy to maintain an organized arrangement of their molecules. When there is no input of energy (making it a “closed system”), chemical bonds may break, large molecules begin to disassemble, and material structures gradually deteriorate. Thus, as a system slowly loses energy, its molecules become more disorganized and randomly distributed. This is an effect of entropy.
en·tro·py
/ˈentrəpē/
1.PHYSICSa thermodynamic quantity representing the unavailability of a system’s thermal energy for conversion into mechanical work, often interpreted as the degree of disorder or randomness in the system.”the second law of thermodynamics says that entropy always increases with time”
2.lack of order or predictability; gradual decline into disorder.”a marketplace where entropy reigns supreme”
The 2nd Law of Thermodynamics or “Entropy” is why terrestrial bodies such as stars die, and why the biological bodies of animals, and humans do not continually evolve upward over time, but devolve and break down over time.
Here are two ways the Theory of Evolution violate the 2nd Law of Thermodynamics:
- Evolution requires that non-living things become more ordered over time to become living things.
- Evolution requires that the least organized species (single celled organisms) become increasingly ordered over thousands and millions of years.
Evolution, the argument goes, is a decrease of entropy, because it involves things getting more organized over time, while the second law says that things get more disordered over time. So evolution violates the second law.
Robert N. Oerter; Professor of Physics & Astronomy; George Mason University
Challenge Question: How can the Theory of Evolution be squared with the 2nd Law of Thermodynamics?
Premise 3 : The Planets, Stars, Sun and Moon should be made of the same chemical elements
Evolutionary theory proposes that our solar system formed from a cloud of swirling gas, dust, and particles.
An extremely dense point exploded with unimaginable force, creating matter and propelling it outward to make the billions of galaxies of our vast universe. Astrophysicists dubbed this titanic explosion the Big Bang
Exploratorium.edu
Supposing evolution’s theory of the “Big Bang” to be true, the planets and their 63 known moons would have evolved from the same “matter” and at the same time under the same conditions. If so then we have to ask why the planets, stars, sun and moon all have different chemical makeups? Why are they often times composed of different matter from each other?
The Composition of Planetary Atmospheres
| Planet | Carbon Dioxide | Nitrogen | Oxygen | Argon | Methane | Sodium | Hydrogen | Helium | Other |
| Sun | 71% | 26% | 3% | ||||||
| Mercury | 42% | 22% | 22% | 6% | 8% | ||||
| Venus | 96% | 4% | |||||||
| Earth | 78% | 21% | 1% | ||||||
| Moon | 70% | 1% | 29% | ||||||
| Mars | 95% | 2.7% | 1.6% | 0.7% | |||||
| Jupiter | 89.8% | 10.2% | |||||||
| Saturn | 96.3% | 3.2% | 0.5% | ||||||
| Uranus | 2.3% | 82.5% | 15.2% | ||||||
| Neptune | 1% | 80% | 19% | ||||||
| Pluto | 8% | 90% | 2% |
This chart shows that the chemical and material makeup of the planets in our solar system are so varied mathematically that the assumption that they all originated at the exact same time from the same matter and energy source seems impossible to maintain scientifically.
Here is a more visual example:
- Imagine if the moon was hollowed out and filled to the brim with bright pink, yellow, and purple paper confetti and then exploded with confetti spreading throughout the milky way galaxy.
- Imagine exploring the galaxy for the exploded confetti and finding some in the Valles Marineris (The Grand Canyon of Mars) and in the Tycho Crater on the moon—shouldn’t the confetti found should be bright pink, yellow, and purple paper confetti? What if it was bright pink, yellow and purple Skittles candy instead?
A primary example of varying chemical makeups is the sun which is composed of 98% hydrogen and helium—yet less that 1% of all the other planets combined mass is hydrogen and helium.
The Moon landings have permitted man to actually study lunar composition and structure. Enough has been found now to permit the firm conclusion that the earth and its moon are of vastly different structure and therefore could not have the same celestial evolutionary “ancestor”.
To the surprise of scientists, the chemical makeup of the moon rocks is distinctly different from that of rocks on earth. This difference implies that the moon formed under different conditions...and means that any theory on the origin of the planets will have to create the earth and the moon in different ways
Jerry E Bishop “New Theories Of Creation,” Science Digest, vol. 72, p. 42
The seven lunar rovers, seven Mars rovers, three asteroid rovers, Hubble telescope, James Webb telescope, satellites, and manned space exploration and all of these technologies have highlighted the major differences in the planets not similarities
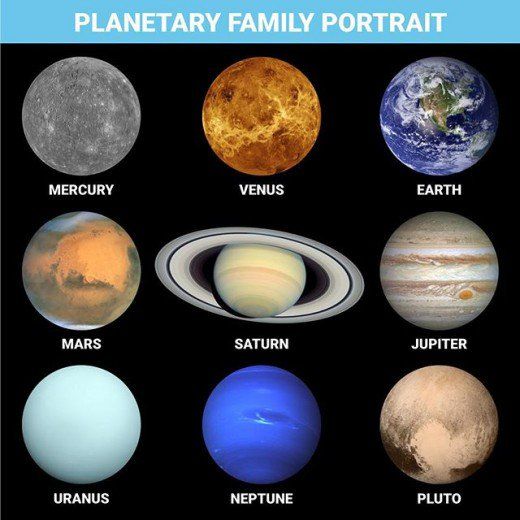
The chemical, physical, and environmental makeups of the planets indicate clear “unrelatability”

Many details as to why Earth is the only planet with liquid water in our solar system need to be worked out
Dana Valencia; Earth and Planetary Sciences Harvard University“We’ve searched carefully for oxygen, carbon, nitrogen and silicon—the things that are found on Earth and the sun in abundance,” Michele Fumagalli, an astronomer at the University of California, Santa Cruz, says in a press release issued by the university. “We don’t find a trace of anything other than hydrogen and deuterium (an isotope of hydrogen).” Michel Fumagalli; Astronomer University of California
If the universe and solar system were created at the same time as the earth, with the same material that the “Big Bang” created the earth from the differences in gases, elements, and metal composition on other planets should not be vastly greater than their similarities.
Challenge Question 1 : If all the planets and stars that exist in the known universe exploded into existence at the same time as the earth, why are the chemical, physical and environmental makeups radically different?
Challenge Question 2: If all the planets in the universe share the same evolutionary birthday and complex life has had the same amount of time to develop there— why hasn’t evolution been detected orreproduced on other planets?
Premise 4: The Law of Abiogenesis states that life cannot originate from non-living matter which evolution requires
The Law of Abiogenesis is credited to the work of Louis Pasteur and others who through repeated scientific experimentation and observation established the axiom— Omne vivum ex ovo “all life is from life.”
Biogenesis describes a process whereby living organisms can only arise from other living organisms. In other words scientist have always adamantly agreed that non living entities such as chemicals, rocks, minerals, or energy cannot produce life.
Spontaneous generation (the emergence of life from nonliving matter) has never been observed. All observations have shown that life comes only from life.
Yet Evolutionist Scientists advocate that the origin of life arose by chance from non-living chemicals through a process called chemical evolution.
However living things get their information from their parent organisms, and contrary to evolutionary doctrine, scientists have never observed life arise from raw, unprogrammed matter.
Most sincere evolutionary scientist admit their is an unexplainable gap in the theory of evolution:
We know how life, once it began, was able to proliferate and diversify until it filled (and in many cases created) every niche on the planet. Yet one of the most obvious big questions–how did life arise from inorganic matter? —Remains a great unknown?
American Scientist, James Trefil Professor of Physics George Mason University
The theory of evolution that says living organisms arose from non-living dust, particles, gases, and chemicals which is called Abiogenesis which is a direct contradiction of the Law Of Biogenesis. Scientist have not been able to replicate Abiogenesis in the laboratory and have never observed non-living matter giving rise to living organisms in nature.
This not only leaves evolutionists without an explanations for not only how life originated from nonlife, but also how non-life became a mechanism for an expanding a more complex gene pool.
Our progress on this question has been impeded by a formidable cognitive barrier. Because we perceive a deep gap when we think about the difference between inorganic matter and life, we feel that nature must have made a big leap to cross that gap. This point of view has led to searches for ways large and complex molecules could have formed early in Earth’s history, a daunting task. The essential problem is that in modern living systems, chemical reactions in cells are mediated by protein catalysts called enzymes. The information encoded in the nucleic acids DNA and RNA is required to make the proteins; yet the proteins are required to make the nucleic acids. Furthermore, both proteins and nucleic acids are large molecules consisting of strings of small component molecules whose synthesis is supervised by proteins and nucleic acids. We have two chickens, two eggs, and no answer to the old problem of which came first.
American Scientist, James Trefil, Harold J. Morowitz, Professors of Physics and Philosophy Stanford and Yale respectfully.
In other words, the matter, gases, and chemicals that supposedly produced life through the big bang could not have provided the genetic information essential to the formation of living things because the components are non-living and the genetic information essential for life does not exist in non-living matter.
Challenge Question : Life coming from life is a cycle that is repeated on earth a billion times a day. Scientists using the most advanced technology have not been able to produce life from non-living matter in the laboratory. Is it therefore reasonable scientist to assertively claim that it has happened before randomly, spontaneously, and undirected?
ThinkCubed Truth Veracity Grid
Have I considered the issue carefully and honestly with an open mind?
Does what I think conform to the rules of logic and avoid contradictions?
Are my conclusions free from bias or presuppositions?
